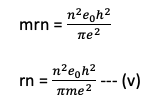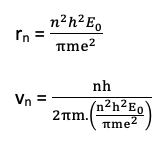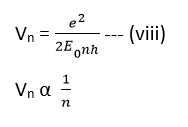Bohr’s atomic model
The basic assumptions of Bohr’s atomic model are:
1. The electron revolves around the nucleus in those orbits for which the angular momentum of the electron is an integral multiple of h/2π, where h = planks constant.
If m is the mass of the electron and v is the velocity of the electron in an orbit of radius ‘r’ then angular momentum.
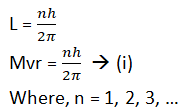
2. Electron does not radiate energy continuously. The atom radiated energy when an electron jump from a higher energy store to a low energy state and energy is absorbed when an electron jumps from a lower energy state to a higher energy state.
If E2 and E1 be the higher energy state and lower energy state respectively then energy gained is given by,
E2 – E1 = hf —(ii)
Bohr’s theory of Hydrogen atom
(a)Radius of the Hydrogen atom
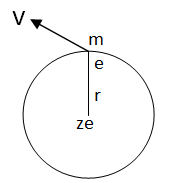
Let us a hydrogen atom that has a positive charge +’ze’ in the nucleus and electron charge (-e) in the figure. Suppose the electron revolving in the n^th orbit whose radius is ‘rn’ with velocity ‘vn’ then according to Columba’s law in electrostatics. The electrostatic force of attraction between the nucleus and electron is given by.
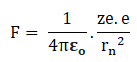
But for the hydrogen atom (z)=1

If m is the mass of an electron the centripetal force acting on the electron is given by
from equations (i) and (ii)
from the first postulate of Bohr’s atomic model we have;

squaring both sides
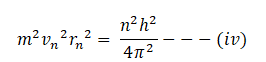
dividing equation (iv) by (iii)
Eqn (v) is used to find the radius of various orbits of the hydrogen atoms.
b) Bohr radius
The radius of the innermost orbit in a hydrogen atom is called Bohr’s radius.
i.e for Bohr’s radius n=1
putting the value on all constant
r1 = 0.529A°
Thus rn =0.529nˆA°
this relation gives the radius of the various orbit.
The velocity of an electron.
From Bohr’s 1st postulate
then, using the value of
This shows that electrons move faster in inner most orbits than in the outer.
The energy of an electron in nth orbit.
The electron is revolving around the nucleus it has kinetics energy and the electron is attached to the nucleus by electron static force of attraction. So it has also potential energy. Thus the total sum of P.E and K.E.

Also the potential energy (P.E) = potential due to nucleus at distance(r) x change of electron
Thus total energy of the electron in the nth orbit is,
En = K.E. + P.E.
The negative sign shows that the election is attached to the nucleus. Also, this equation shows that if ‘n’ increases ‘En’ increases the outer orbit has more energy than the inner orbit.
Bohr’s interpretation of the Hydrogen spectra
If an electron jumps from a higher energy state of orbit ‘n2’ to the lower energy state of orbit ‘n1’ then the frequency of emitted radiation is,
Where En1 + En2 is the energy of an electron in orbit n1 + n2.
Now we have,
The reciprocal of wavelength gives the wave number contained in the radiation
where
is called Rydberg constant
Equation (iii) gives the series of hydrogen spectra called spectral series.
Spectral series of the hydrogen atom
When an electron in hydrogen atoms jumps from a higher energy level to a lower energy level radiations of a particular wavelength are emitted this is called a spectral line. A spectral line has similar property spectral series the spectral series are:
1. Lymen series
2. Balmer series
3. Paschen series
4. Bracket series
5. P-fund series
1. Lymer Series
Lymer series is obtained when the excited electrons jump back to the first energy level (n1 = 1) from a higher energy level n2 (2, 3, 4, 5…)
The wave number and wavelength corresponding to lymer series are
this series lies in the ultra region.
Balmer Series
Balmer series is obtained when the excited electron jumps back to the second energy level (n=2) from a higher energy level (n = 3, 4, 5, 6..)
The wave number and wavelength are
this series on the visible region
Paschen Series
Paschen series is obtained when the excited electron jumps back to the third energy level (n1 = 3) from a higher energy level (n = 4, 5, 6..)
The wave number and wavelength corresponding to the paschal series are
this series lies in the infrared region.
Bracket series
The bracket series is obtained when the excited electron dump back to the fourth energy level (n1=4) from a higher energy level n2 = (5,6,7,–)
The wave number and wavelength corresponding to the Bracket series are
This series lies in the infrared region.
P-fund series
p-fund series obtained when the excited electron jump back to the fifth energy level (n1=5) from a higher energy level n2=(6,7,8_ _ _)
the wave number and wavelength corresponding to the p-fund series are
It lies in the far-infrared region.

Energy level diagram of a hydrogen atom
The energy of the electron in the nth orbit is given by,
Equation (i) can be diagrammatically represented which is called the energy level diagram.
Putting the value of, e, E0, h we get,
for n=1, we get
E1 = -13.6/1^2 = -13.6 ( It is called the energy of the first excited state of the hydrogen atom)
Similarly ,
E2 = -13.6/2^2 =-3.4ev
E3 = -13.6/3^2 = -1.5ev
E4 = -13.6/4^2 = -0.85ev
E5 = -13.6/5^2 = -0.54ev
Also,
Excitation energy
The minimum energy required to jump an electron from the ground state to any one of the excitation states is called excitation energy.
If Eg is the energy corresponding to the ground state and Ei is the energy of the excitation state the,
excitation energy = Ei – Eg
for eg:
In a hydrogen atom for an electron from the ground state to 1st excited state the required energy is,
[Ei – Eg]
Ei – Eg
[-3.4 – (-13.6)]ev
10.2ev first excitation energy
thus 10.2ev energy is required to jump from 2nd to 1st state
The potential which is required for an electron to jump from the ground state to any excited state is called excitation potential.
The excitation potential for 1st excited state of a hydrogen atom is 10.2ev and 2nd excitation potential of a hydrogen atom is 12.19v and soon.
Ionization energy
The minimum energy of energy required to remove an electron completely from an atom is called ionization energy.
for eg:
To remove an electron from a hydrogen atom the energy required is
This shows that
Numerically ground state energy equal to ionization energy.
The potential corresponding to ionization energy is called ionization potential.
Limitation of Bohr’s Model
a. It explains only hydrogen spectra but fails to explain spectra of the multielectron atoms.
b. It cannot explain the intensity of the spectral line.
c. It fails to explain the wave nature of the electron.
d. It fails to explain the elliptical orbit of the electron.
De-Broglie Theory
According to De-Broglie’s theory, a moving particle sometimes behaves like a wave as well as a particle.
The de-Broglie wavelength of an electron
Since for electron
This is the required relation for the De-Broglie wavelength of an electron.
Heisenberg’s uncertainty principle
According to Heisenberg’s uncertainty principle, it is impossible to measure any two canonically conjugate quantities simultaneously with absolute accuracy. That means if one of them is measured with accuracy then the other becomes less accurate.
For example:
Two economically conjugate quantities’ positions and momentum cannot be determined simultaneously. If ∆x and ∆P are the uncertainty of position and momentum then,
If Δx—>0 then Δp—>∞
and
If Δp—>0 then Δx—>∞
Equation (i) is Heisenberg’s uncertainly principle for position and momentum
similarly, if ΔE and Δt are uncertainties of energy and time then,
ΔEΔt ≥h—(ii) when h= h/2π
Equation(ii) is the uncertainty principle for energy and time
X-ray
When fast-moving electrons strike a target of high melting point and high atomic weight like tungsten, platinum, or molybdenum, X-rays are produced.
Properties of X-rays
1) X-rays are the electromagnetic wave of short wavelength (10^-9 – 10^-12)
2) They travel in a vacuum with a speed of high (3×10^8 m/s)
3) They have a high penetrating power.
4) They affect a photographic plate
5) In Similar light, x-ray produces a photoelectric effect
6) They have a destructive effect on living tissue
7) They are not deflected by the electric and magnetic field
Production of x-rays (Modern collage tube)

Fig: Modern college tube
A modern college tube consists of target metal ‘T’ and cathode which are enclosed inside (+ve) evaluated glass tube ad shown in the figure above. A target metal is made with metal having a high melting point and high atomic weight like tungsten platinum. A low tension battery is used in order to heat the cathode from which beam of electron on target produces heat. A copper tube is placed along the side of the tungsten metal wire.
When power is switched on the cathode is heated and emits electrons there electrons are allowed to strike a target metal having a high melting point and high atomic weight. Due to this, the atom of the target metal gets excited to a higher energy level, and when the excited atom return to its ground state x-rays are emitted.
About 98% of energy is converted into heat and the remaining 2% come out as x-rays. This large amount of heat can melt the target metal. So a connecting tube is placed alongside the target metal to control melting.
2dsinθ = nλ
Some terms
lattice plane and lattice constant (d)
lattice constant (crystal / spacing)(d)
Bragg’s law
When the path between two reflected x-rays from two different planes of crystals is equal to an integral multiple of the wavelength of incident x-rays, the intensity of the reflected beam of x-ray at a certain angle will be maximum i.e.
The intensity of a reflected beam of x-ray will be maximum when,
Path difference = n λ
Where n = 1, 2, 3, …

Let us consider two lattice planes x, y, and x1 and y1 with lattice constant ‘d’ (crystal spacing) as shown in the figure above. Also, consider two parallel x-rays PQ and P’Q’ are incidents on two planes XY and x’y’ at a glancing angle ‘θ’ and along QR and Q’R’ from the atom Q and Q’ respectively.
In order to calculate the path difference between two reflected rays, draw perpendicular QT on P’Q’ and QS on Q’R’.
Then path difference between two reflected rays = TQ’ and Q’S
Now, at the right angle ∆QTQ’
∴ Path difference = TB’ + B’Sθ
= dsinθ + dsinθ
= 2dsinθ
But for maximum intensity
Path difference = integral multiple of the wavelength
2dsinθ = nλ
Diffraction of X-rays (lava’s Experiment)

The diffraction of x-rays shown in Lava’s experiment this experimental arrangement is shown above. where ‘s’ is the surface of x-rays from which a continuous range of x-rays are obtained through two slits S1 and S2 a narrow beam of x-rays is passed towards Zns (zinc sulfide) crystal and finally x-rays beam fall on a photographic plate
When the photographic plate is developed for image, many concentric spots are observed around a central back spot obtained in the photographic plate is known as lava’s spot this is shown x-ray is diffracted from Zns crystal
From this experiment, it can conclude that
1) X-rays are the electromagnetic wave of very short wavelength
2) The atoms of the crystal are arranged in a regular three-dimensional lattice
If you want to ask anything, please contact us by Messaging us on Social Media.
Class 12 Physics Notes 2078: Complete Physics Guide (New Syllabus) | Questions Answers and Guide | Class 12 Physics Book Solution Guide | Class 12 Physics Book PDF 2078 Solution | Class 12 Physics Book Notes 2078 Guide | Class 12 Physics All Chapter Notes | Physics Chapter Wise Notes. Class 12 Physics Quantization of energy Notes.